Green Stone > Products > Pharmaceutical Ingredients > Views
Dexlansoprazole Reference standard
[
customers have already purchased this product.]
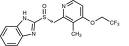
Product name : Dexlansoprazole Reference standard
CAS : 138530-94-6
Molecular formula : C16H14F3N3O2S
Molar mass:369.363
Product description :
Dexlansoprazole (INN, trade names Kapidex, Dexilant) is a proton pump inhibitor that is marketed by Takeda Pharmaceuticals. Chemically, it is an enantiomer of lansoprazole. The compound was launched in the US for use in the treatment and maintenance of patients with erosive oesophagitis and non-erosive gastro-oesophageal reflux disease (GERD or GORD). Dexlansoprazole was approved by the U.S. Food and Drug Administration (FDA) on January 30, 2009.
Category

Recommend products






 Asia Bio-Pharmaceutical Research Institute
Asia Bio-Pharmaceutical Research Institute  Green Stone Swiss Co ., Ltd.
Green Stone Swiss Co ., Ltd.


Send to this supplier
1. Email: sales@Greenstoneswiss.com
2. Tel: +86 592 5365887
3. WhatsApp: +86 189 6515 7632
4. Send enquiry online:
Customer also maybe viewed the follwing products
Product Image
Product Name
Calcium sulfate
Pyridostigmine Deuterated
Lauryl glucoside
Agrimory Extract
CAS NO.
Order